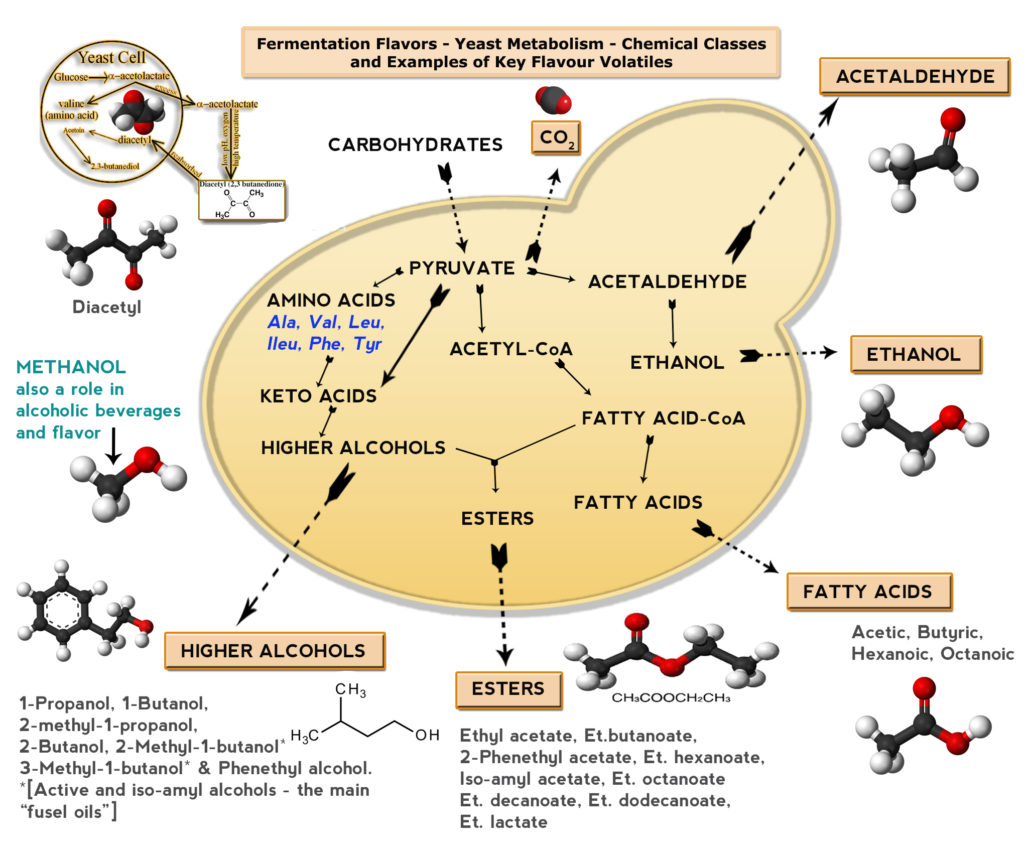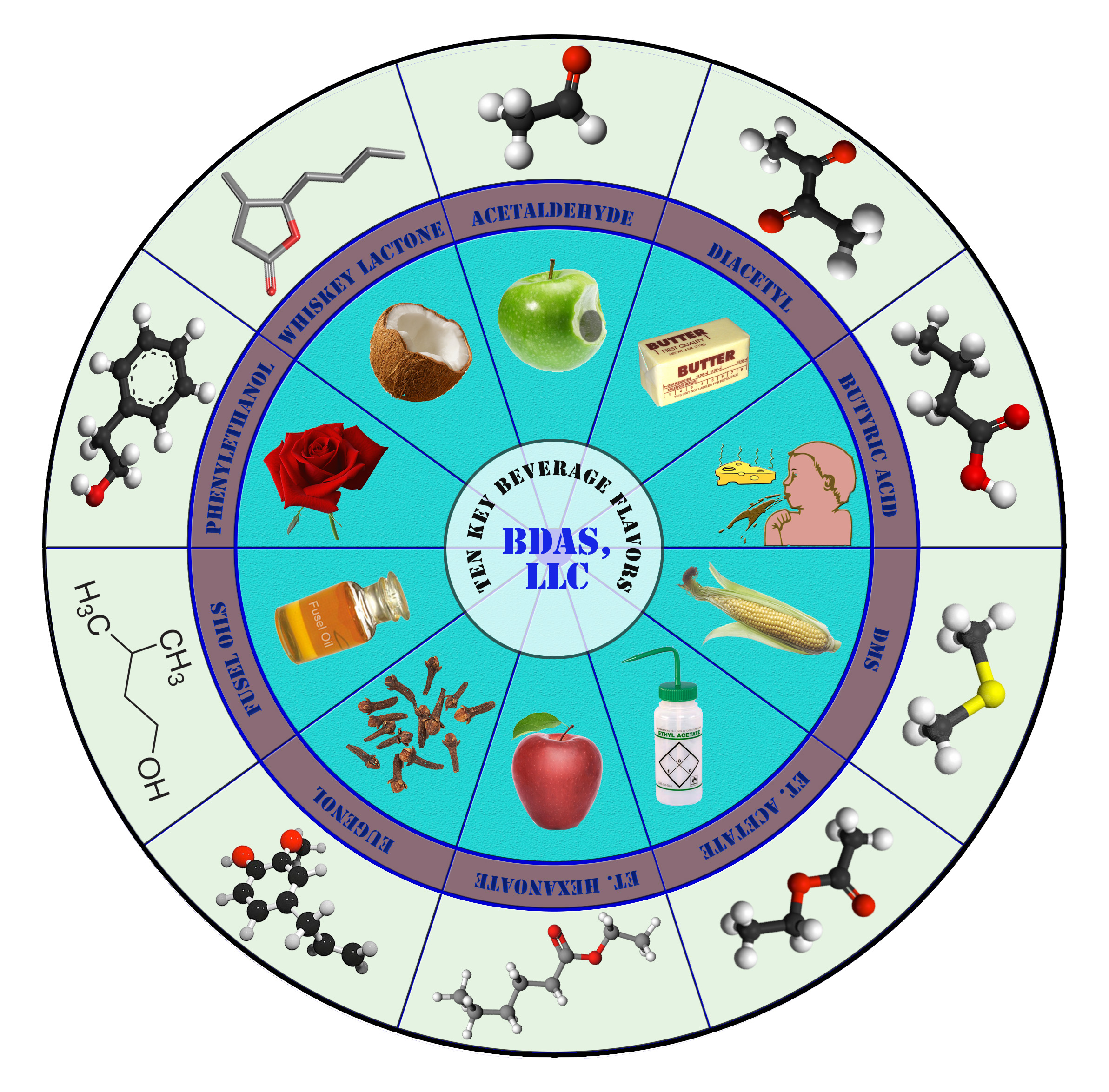
From a chemists viewpoint all classes of compounds will be present, alcohols (ethanol and higher – meaning longer carbon chain length – alcohols – sometimes called fusel oils – with solventy, nutty and even roses-like notes) aldehydes (green apple-like acetaldehyde, almond-marzipan from benzaldehyde), ketones (the dreaded buttery compound diacetyl), esters (fruity and solventy notes – think bananas in wheat beers or red apple and hints of aniseed or tropical fruit notes), phenols (cloves in wheat beers), acids (acetic and lactic acid and more complex acids leading to cheesy, rancid, goaty notes), sulfur compounds (dimethyl sulfide – cooked corn, tomatoes, oysters – the ocean spray, hydrogen sulfide – rotten eggs, hair permanent solution, or sulfites – burnt matches) and more create about a third or more of over a 1000 components in your glass.
Some vying for full attention, some quietly lurking in the background but all providing what makes beer – well essentially beer! See Figure 2 for a very brief overview graphic covering the keys to fermentation flavour production via yeast metabolism.